Hilma Biocare: The Ultimate 2025 B2B Sourcing Guide
Introduction: Navigating the Global Market for hilma biocare
In today’s dynamic pharmaceutical landscape, sourcing high-quality products from companies like Hilma Biocare poses unique challenges for international B2B buyers. With a growing demand for effective and affordable medications, especially in regions such as Africa, South America, the Middle East, and Europe, understanding the intricacies of Hilma Biocare’s offerings is crucial. This comprehensive guide will delve into the diverse range of products available, including injectables, oral tablets, and advanced formulations like peptides and hormone therapies.
Moreover, it will address essential aspects such as supplier vetting, compliance with global quality standards, and the cost structures that impact purchasing decisions. By providing detailed insights into product applications and market trends, this guide aims to equip B2B buyers with the knowledge needed to make informed choices, ensuring that they can confidently navigate the complexities of sourcing from Hilma Biocare.
As you explore the various sections, you will uncover actionable strategies to optimize your procurement processes, enhance your supply chain reliability, and ultimately contribute to better health outcomes in your respective markets. With Hilma Biocare’s commitment to quality and innovation, this guide serves as an indispensable resource for businesses looking to thrive in the competitive pharmaceutical market.
Understanding hilma biocare Types and Variations
| Type Name | Key Distinguishing Features | Primary B2B Applications | Brief Pros & Cons for Buyers |
|---|---|---|---|
| Injectable Products | Available in various forms (e.g., vials, pens) | Hospitals, clinics, and pharmacies | Pros: Quick effects, high bioavailability. Cons: Requires proper storage and administration. |
| Oral Tablets | Easy to administer, suitable for long-term use | Pharmacies, health retailers | Pros: Convenient for patients, stable shelf life. Cons: Slower onset of action compared to injectables. |
| Hormonal Therapies | Includes testosterone and growth hormone formulations | Endocrinology and sports medicine | Pros: Effective for specific hormonal deficiencies. Cons: Regulatory scrutiny and potential side effects. |
| Peptide Products | Targeted therapies for various conditions | Research institutions and specialized clinics | Pros: Specificity in action, minimal side effects. Cons: Complexity in sourcing and usage regulations. |
| Post Cycle Therapy (PCT) | Designed to restore hormonal balance after steroid use | Bodybuilding and fitness centers | Pros: Essential for recovery, improves overall health. Cons: May not be widely understood outside fitness circles. |
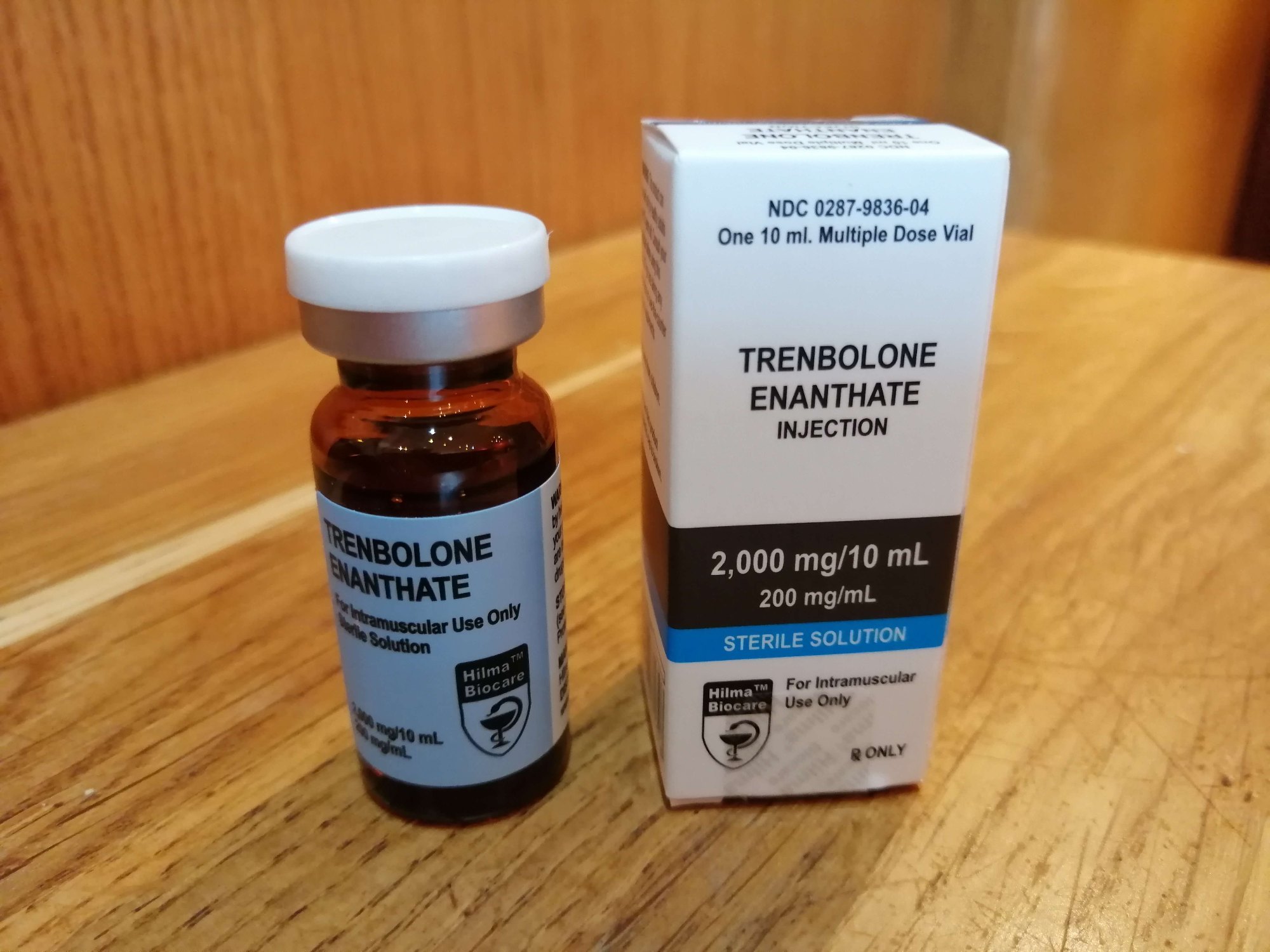
What Are the Key Characteristics of Hilma Biocare’s Injectable Products?
Injectable products from Hilma Biocare are designed for rapid absorption and effectiveness. They come in various forms, including traditional vials and the more modern liquid pens, which offer ease of use and precise dosing. These products are primarily utilized in hospitals and clinics, making them essential for immediate medical interventions. B2B buyers should consider storage requirements and proper administration techniques, as these factors are crucial for maintaining product integrity and ensuring patient safety.
How Do Oral Tablets Stand Out in Hilma Biocare’s Offerings?
Oral tablets are a cornerstone of Hilma Biocare’s product line, favored for their convenience and ease of use. These products cater to patients who prefer non-invasive treatment options, making them popular in pharmacies and health retailers. They typically have a longer shelf life compared to injectables, which can be advantageous for inventory management. However, B2B buyers should be aware that the onset of action is slower, which may not be suitable for all clinical situations.
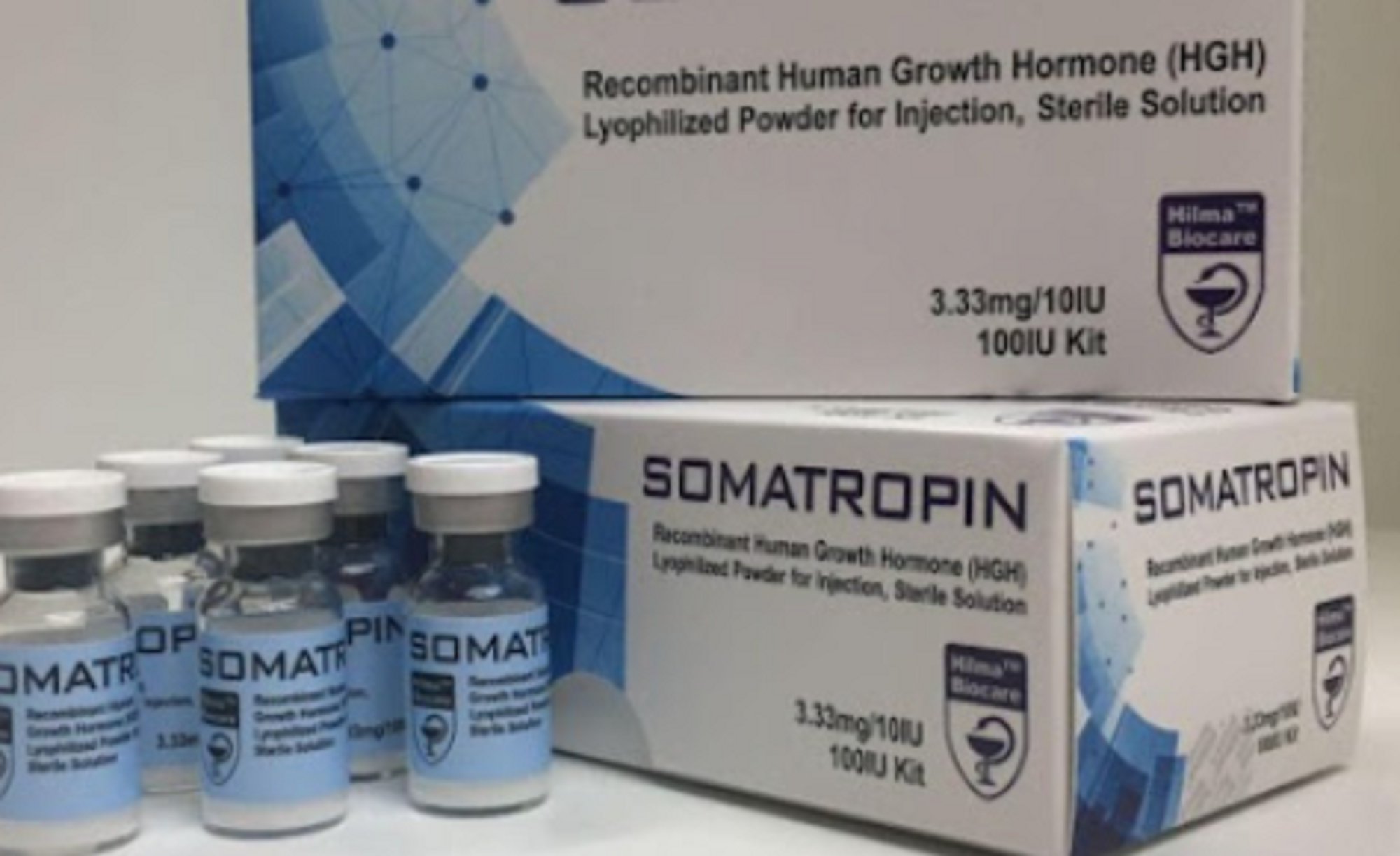
What Should B2B Buyers Know About Hormonal Therapies?
Hilma Biocare’s hormonal therapies, including testosterone and growth hormone products, are specifically formulated to address hormonal deficiencies. These products are primarily used in endocrinology and sports medicine, where they can significantly enhance patient quality of life. B2B buyers must navigate regulatory considerations and potential side effects, ensuring that they are well-informed about the therapeutic applications and patient management strategies associated with these products.
Why Are Peptide Products Important for Specialized Clinics?
Peptide products from Hilma Biocare offer targeted therapies for a variety of medical conditions, appealing to research institutions and specialized clinics. Their specificity allows for minimal side effects, making them an attractive option for practitioners seeking effective treatment modalities. However, sourcing these products can be complex due to regulatory challenges, and B2B buyers should have a clear understanding of the legal landscape surrounding peptide usage.
How Does Post Cycle Therapy (PCT) Fit into Hilma Biocare’s Product Range?
Post Cycle Therapy (PCT) products are crucial for individuals recovering from steroid use, particularly in bodybuilding and fitness circles. They help restore hormonal balance, contributing to overall health and well-being. While these products are essential for recovery, they may not be widely understood outside specific fitness communities. B2B buyers should ensure they are equipped with educational resources to effectively communicate the benefits and importance of PCT to their clients.
Key Industrial Applications of hilma biocare
| Industry/Sector | Specific Application of hilma biocare | Value/Benefit for the Business | Key Sourcing Considerations for this Application |
|---|---|---|---|
| Pharmaceuticals | Development of generic medications | Cost-effective treatment options for patients | Compliance with GMP standards and local regulations |
| Sports and Fitness | Anabolic steroids and performance enhancers | Improved athletic performance and recovery | Quality assurance and product testing documentation |
| Healthcare Institutions | HGH and peptide therapies | Enhanced patient outcomes in growth and recovery | Reliable supply chain and product efficacy data |
| Retail and E-commerce | Distribution of wellness and supplement products | Diverse product range attracting various customer segments | Competitive pricing and shipping logistics |
| Veterinary Medicine | Hormonal therapies for animals | Improved health and productivity in livestock | Regulatory compliance and veterinary approval |
How Are hilma biocare Products Used in Pharmaceuticals?
In the pharmaceutical sector, hilma biocare specializes in the development of generic medications, which are crucial for providing affordable treatment options. This helps address the rising healthcare costs faced by many countries, particularly in Africa and South America. International buyers must ensure that the products meet local regulatory requirements and are manufactured under Good Manufacturing Practices (GMP) to guarantee safety and efficacy.
What Role Does hilma biocare Play in Sports and Fitness?
Hilma biocare’s anabolic steroids and performance enhancers are popular among athletes and fitness enthusiasts. These products can significantly improve performance, recovery times, and muscle growth. For B2B buyers in this sector, it’s essential to consider quality assurance, as well as the legal implications of distributing such substances in their respective markets, particularly in Europe where regulations may vary.
How Does hilma biocare Support Healthcare Institutions?
Healthcare institutions utilize hilma biocare’s Human Growth Hormone (HGH) and peptide therapies to improve patient outcomes, particularly in cases of growth deficiencies and recovery from illness or surgery. Buyers in this sector should focus on sourcing products that have undergone rigorous clinical testing and are backed by scientific evidence to ensure the best possible care for patients.
What Are the Benefits of hilma biocare Products for Retail and E-commerce?
Retailers and e-commerce platforms benefit from hilma biocare’s diverse range of wellness and supplement products, appealing to a broad customer base. This variety not only attracts different segments of consumers but also enhances overall sales potential. Buyers should prioritize competitive pricing and efficient shipping logistics to maintain a profitable operation while meeting customer demand.
How Are hilma biocare Products Applied in Veterinary Medicine?
In the veterinary sector, hilma biocare offers hormonal therapies that can improve the health and productivity of livestock. These treatments can lead to better growth rates and overall animal wellness, which is vital for farmers and agricultural businesses. Buyers must ensure that the products are compliant with veterinary regulations in their region to avoid legal issues and ensure animal safety.
3 Common User Pain Points for ‘hilma biocare’ & Their Solutions
Scenario 1: Navigating Quality Assurance in Product Sourcing
The Problem: B2B buyers often face uncertainty regarding the quality and safety of pharmaceutical products, especially when sourcing from international suppliers like Hilma Biocare. With the proliferation of counterfeit drugs and inconsistent quality standards across regions, buyers can feel anxious about the reliability of the products they are purchasing. This concern is particularly heightened for companies operating in regions with stringent regulatory requirements, as they cannot afford to compromise on quality.
The Solution: To mitigate these concerns, B2B buyers should prioritize establishing a strong relationship with Hilma Biocare. Initiate direct communication with their sales and quality assurance teams to understand their manufacturing processes, quality control measures, and compliance with Good Manufacturing Practices (GMP). Request detailed documentation such as Certificates of Analysis (CoA) for specific products, which provide transparency about the product’s quality and composition. Additionally, consider participating in or arranging site visits to their production facilities if feasible. This approach not only reassures you about the quality but also demonstrates a commitment to a long-term partnership.
Scenario 2: Managing Regulatory Compliance Across Borders
The Problem: Different countries have varied regulations concerning the importation and distribution of pharmaceutical products. B2B buyers may struggle to navigate these complex legal landscapes, especially when importing products like those offered by Hilma Biocare into regions with strict pharmaceutical regulations, such as the European Union or South Africa. Non-compliance can lead to severe penalties, product recalls, or damage to the company’s reputation.
The Solution: To effectively manage regulatory compliance, buyers should engage local regulatory consultants or legal advisors familiar with the pharmaceutical landscape in their respective countries. They can assist in understanding specific requirements related to product registration, labeling, and importation. Additionally, maintain open lines of communication with Hilma Biocare’s regulatory affairs team. They can provide guidance on how to meet local regulations and may offer necessary documentation to facilitate the compliance process. Consider developing a compliance checklist tailored to your market, ensuring all legal requirements are met before placing orders.
Scenario 3: Addressing Supply Chain Disruptions
The Problem: Supply chain disruptions, whether due to global events, logistics issues, or sudden changes in demand, can create significant challenges for B2B buyers of Hilma Biocare products. Delays in receiving critical pharmaceuticals can impact not only operational efficiency but also patient health outcomes in healthcare settings.
The Solution: To counter supply chain disruptions, B2B buyers should diversify their sourcing strategies by establishing a network of reliable suppliers, including Hilma Biocare. Engage in proactive inventory management practices, such as maintaining safety stock levels for essential products. Utilize demand forecasting tools to better anticipate needs based on historical sales data and market trends. Moreover, consider implementing an agile supply chain model that allows for quick responses to changes in demand or supply availability. Regularly review and update contracts with suppliers to include clauses that address potential disruptions, ensuring that contingency plans are in place for unexpected scenarios. By taking these steps, buyers can build resilience into their supply chains, minimizing the impact of disruptions.
Strategic Material Selection Guide for hilma biocare
What Are the Key Materials Used in Hilma Biocare Products?
In the pharmaceutical industry, the selection of materials is critical to ensuring product efficacy and safety. Hilma Biocare, known for its commitment to quality and innovation, utilizes various materials in its product formulations and packaging. Below are analyses of four common materials used by Hilma Biocare, focusing on their properties, advantages, disadvantages, and implications for international B2B buyers.
What Are the Key Properties of Glass in Pharmaceutical Applications?
Glass is a primary material used for packaging injectable medications. Its key properties include excellent chemical resistance and impermeability to gases and moisture, which are crucial for maintaining the integrity of pharmaceutical products. Glass can withstand high temperatures during sterilization processes, making it suitable for various applications.
Pros: Glass is highly durable and provides an inert environment that does not react with the drug. It also offers excellent visibility of the contents, which is beneficial for quality control.
Cons: The fragility of glass can lead to breakage during transportation and handling. Additionally, glass packaging tends to be heavier, which can increase shipping costs.
Impact on Application: Glass is compatible with a wide range of pharmaceutical media, including sensitive compounds that require stable storage conditions.
Considerations for International Buyers: Buyers from regions such as Africa and South America should ensure compliance with international standards like ISO 8362 for glass vials. Familiarity with local regulations regarding glass packaging is also essential.
How Does Polypropylene Contribute to Pharmaceutical Packaging?
Polypropylene (PP) is increasingly used in the production of pharmaceutical containers and closures. Its key properties include a high melting point and good chemical resistance, making it suitable for various pharmaceutical applications.
Pros: PP is lightweight and less prone to breakage compared to glass, which can reduce shipping costs and improve safety during handling. It is also cost-effective, making it an attractive option for manufacturers.
Cons: While PP has good chemical resistance, it may not be suitable for all types of drugs, particularly those that are sensitive to leachables from plastics.
Impact on Application: PP is widely used for oral medications and some injectables, but its compatibility with specific drug formulations should be evaluated.
Considerations for International Buyers: Buyers should be aware of compliance with regulations such as the FDA’s Title 21 CFR for plastic materials in contact with pharmaceuticals. Understanding local preferences for packaging materials is also vital.
What Role Does Aluminum Play in Pharmaceutical Products?
Aluminum is commonly used for blister packs and closures in the pharmaceutical industry. Its key properties include lightweight, corrosion resistance, and excellent barrier properties against moisture and light.
Pros: Aluminum packaging is highly effective in preserving the stability and shelf life of pharmaceutical products. It is also recyclable, aligning with sustainability goals.
Cons: Aluminum can be more expensive than other materials, and its manufacturing process can be complex, potentially leading to higher production costs.
Impact on Application: Aluminum is ideal for moisture-sensitive products, ensuring that the medication remains effective throughout its shelf life.
Considerations for International Buyers: Buyers should consider compliance with relevant standards such as ASTM B209 for aluminum sheets and foils. Awareness of local regulations regarding recyclable materials can also influence purchasing decisions.
How Does Rubber Contribute to Pharmaceutical Packaging?
Rubber is often used in the formulation of stoppers and seals for vials. Its key properties include elasticity and a good sealing capability, which are essential for maintaining sterility in pharmaceutical products.
Pros: Rubber stoppers provide an excellent seal that helps prevent contamination. They are also flexible, allowing for easy access to the medication.
Cons: Rubber can be susceptible to degradation over time, especially when exposed to certain solvents or temperatures, which may compromise the integrity of the seal.
Impact on Application: Rubber is compatible with a variety of injectable products, but it is essential to evaluate the specific rubber type used to ensure it does not interact with the drug.
Considerations for International Buyers: Buyers should ensure that rubber components comply with regulations such as ISO 8362-1 for rubber stoppers. Understanding the specific needs of the target market regarding rubber formulations is also crucial.
Summary Table of Material Selection for Hilma Biocare
| Material | Typical Use Case for hilma biocare | Key Advantage | Key Disadvantage/Limitation | Relative Cost (Low/Med/High) |
|---|---|---|---|---|
| Glass | Injectable medications packaging | Excellent chemical resistance | Fragile, heavier than alternatives | Medium |
| Polypropylene | Oral tablets and some injectables | Lightweight, cost-effective | Limited compatibility with some drugs | Low |
| Aluminum | Blister packs and closures | Good barrier properties | Higher manufacturing complexity | High |
| Rubber | Stoppers and seals for vials | Excellent sealing capability | Susceptible to degradation | Medium |
This strategic material selection guide provides valuable insights for international B2B buyers, enabling them to make informed decisions when sourcing materials for pharmaceutical products from Hilma Biocare.
In-depth Look: Manufacturing Processes and Quality Assurance for hilma biocare
What Are the Key Stages in the Manufacturing Process at Hilma Biocare?
Hilma Biocare employs a comprehensive manufacturing process designed to ensure the production of high-quality pharmaceutical products. The manufacturing process can be broken down into four main stages: material preparation, forming, assembly, and finishing.
-
Material Preparation: This initial stage involves the careful selection and testing of raw materials to ensure they meet stringent quality standards. Each ingredient is subjected to thorough inspection and testing to verify its purity and efficacy. This step is crucial as it lays the foundation for the quality of the final product.
-
Forming: During the forming stage, the prepared materials are processed into the desired form. This may include the formulation of tablets, injections, or other pharmaceutical products. Advanced techniques such as blending, granulation, and compounding are utilized, employing state-of-the-art machinery to achieve precise dosages and uniformity.
-
Assembly: In the assembly phase, the individual components of the products are brought together. For injectable products, this includes filling vials or syringes under sterile conditions. The assembly process is meticulously controlled to prevent contamination and ensure the integrity of the product.
-
Finishing: The final stage involves packaging and labeling the products according to regulatory requirements. Hilma Biocare uses advanced packaging technologies, including new security seals and dark glass vials, to enhance product stability and safety. The finishing process also includes final quality checks to ensure that all products meet the required specifications before they are released to the market.
How Does Hilma Biocare Ensure Quality Control Throughout the Manufacturing Process?
Quality control (QC) is integral to Hilma Biocare’s manufacturing philosophy. The company adheres to international quality standards such as ISO 9001, as well as industry-specific regulations including CE and Active Pharmaceutical Ingredients (API) guidelines. Here’s how quality is maintained throughout the manufacturing process:
-
Quality Control Checkpoints: Hilma Biocare implements several QC checkpoints at various stages of production:
– Incoming Quality Control (IQC): Raw materials undergo rigorous testing upon arrival to ensure they meet predefined quality criteria.
– In-Process Quality Control (IPQC): Continuous monitoring is conducted during the manufacturing process to identify any deviations from quality standards in real-time.
– Final Quality Control (FQC): Once the products are completed, they undergo a final inspection that includes visual checks, stability testing, and efficacy assessments. -
Testing Methods: Common testing methods employed by Hilma Biocare include:
– Chemical Analysis: Techniques such as High-Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC) to assess the composition of active ingredients.
– Microbial Testing: To ensure sterility and absence of harmful microorganisms, particularly for injectable products.
– Stability Testing: Conducted to determine the shelf life and storage conditions for products, ensuring they remain effective until the end of their shelf life.
What Steps Can B2B Buyers Take to Verify the Quality Control Measures of Hilma Biocare?
For B2B buyers, especially those from regions like Africa, South America, the Middle East, and Europe, verifying a supplier’s quality control measures is essential. Here are actionable steps buyers can take:
-
Conduct Supplier Audits: Prospective buyers should consider conducting on-site audits of Hilma Biocare’s manufacturing facilities. This allows for direct observation of the processes and adherence to quality standards.
-
Request Quality Assurance Documentation: Buyers should request comprehensive documentation detailing the quality assurance processes and certifications Hilma Biocare possesses. This includes ISO certificates, GMP compliance records, and results from recent quality audits.
-
Engage Third-Party Inspectors: Utilizing third-party inspection services can provide an objective assessment of Hilma Biocare’s manufacturing processes. These inspections can cover everything from raw material sourcing to final product testing.
-
Review Testing Reports: Buyers should ask for access to testing reports that demonstrate the results of IQC, IPQC, and FQC. This documentation can provide insights into the consistency and reliability of the products.
What Are the Unique Quality Assurance Considerations for International B2B Buyers?
International B2B buyers must navigate various quality assurance nuances that could affect their purchasing decisions. Here are some considerations to keep in mind:
-
Regulatory Compliance: Different countries have varying regulations regarding pharmaceutical products. Buyers from regions such as Africa and South America should ensure that products comply with local regulatory standards while also meeting international quality benchmarks.
-
Product Serialization: In regions like Europe, product serialization is mandatory. Buyers should confirm that Hilma Biocare’s products are adequately serialized to facilitate traceability and compliance with local laws.
-
Cultural and Market Differences: Understanding the specific healthcare needs and purchasing capabilities of different regions is crucial. Hilma Biocare’s commitment to producing affordable medications is a significant advantage for buyers in developing markets.
-
Potential for Supply Chain Disruptions: International buyers should also assess the stability of the supply chain and the potential impact of geopolitical factors on product availability and pricing.
By carefully examining these aspects of manufacturing processes and quality assurance, B2B buyers can make informed decisions when partnering with Hilma Biocare, ensuring they receive high-quality pharmaceutical products that meet their specific needs.
Practical Sourcing Guide: A Step-by-Step Checklist for ‘hilma biocare’
This guide aims to assist international B2B buyers in effectively sourcing products from Hilma Biocare. By following this step-by-step checklist, you will ensure a streamlined procurement process while adhering to quality standards and optimizing your purchasing decisions.
Step 1: Identify Your Product Needs
Before initiating contact with suppliers, clearly define what products you require from Hilma Biocare. This includes specifying the types of pharmaceuticals, such as injectables, oral tablets, or peptides. Understanding your needs will help in evaluating suppliers who can meet your specific demands and ensuring that they have the necessary inventory.
Step 2: Research Supplier Credentials
Verify the credentials and reputation of potential suppliers. Look for certifications such as Good Manufacturing Practice (GMP) compliance, which ensures that products meet quality standards. Additionally, check their operational history, including the number of countries served and years in business, to gauge reliability.
Step 3: Evaluate Product Quality
Investigate the quality of Hilma Biocare products by requesting samples or detailed product specifications. Ensure that the products have undergone clinical testing and adhere to safety regulations. Pay attention to packaging and labeling, as these factors can impact product integrity and compliance with local laws.
Step 4: Assess Pricing and Payment Terms
Request detailed pricing information and compare it with other suppliers. Consider not only the unit price but also potential discounts for bulk purchases. Evaluate the payment terms offered, including payment methods and any upfront costs, to ensure they align with your budget and financial practices.
Step 5: Inquire About Shipping and Delivery Options
Discuss logistics with the supplier, focusing on shipping times, methods, and costs. Understand the delivery process, including tracking options and handling of customs duties, especially if sourcing from international locations. Reliable shipping practices are crucial for maintaining your supply chain efficiency.
Step 6: Seek Customer References and Reviews
Request references from existing clients or seek out customer reviews online. This step is vital for assessing the supplier’s reliability and customer service quality. Look for feedback on delivery times, product satisfaction, and overall experience to gauge how well they meet their commitments.
Step 7: Establish Clear Communication Channels
Ensure that you have established communication lines with your chosen supplier. Discuss preferred communication methods, response times, and the main point of contact. Effective communication is key to resolving any issues quickly and maintaining a smooth procurement process.
By following this checklist, you can confidently navigate the sourcing process for Hilma Biocare products, ensuring that you select a supplier that meets your business needs while upholding quality and reliability.
Comprehensive Cost and Pricing Analysis for hilma biocare Sourcing
What Are the Key Cost Components in Sourcing Hilma Biocare Products?
When analyzing the cost structure for sourcing products from Hilma Biocare, several key components come into play:
-
Materials: The cost of raw materials is critical, especially for pharmaceutical products where quality and sourcing can greatly affect price. Hilma Biocare emphasizes high-quality ingredients, which can lead to higher initial costs but ultimately ensures product efficacy and safety.
-
Labor: The workforce involved in production, from skilled pharmacists to assembly line workers, contributes to labor costs. Given that Hilma Biocare operates under GMP standards, labor costs may reflect a premium for trained personnel who adhere to strict quality controls.
-
Manufacturing Overhead: This includes costs related to facilities, utilities, and equipment maintenance. For Hilma Biocare, maintaining high-quality production standards necessitates significant investment in advanced manufacturing technologies and facilities.
-
Tooling: Initial setup and tooling costs can be substantial, particularly for specialized products. Custom formulations may require unique production setups, which can impact the overall cost structure.
-
Quality Control (QC): Rigorous QC measures are essential in the pharmaceutical industry. Hilma Biocare’s commitment to clinical testing and compliance with regulatory standards adds an additional layer of cost but is crucial for ensuring product safety and efficacy.
-
Logistics: Distribution costs, including shipping and handling, can vary significantly based on the destination. With Hilma Biocare serving a diverse international market, logistics costs must be carefully evaluated, particularly for buyers in regions like Africa and South America, where infrastructure may impact delivery times and costs.
-
Margin: Finally, the profit margin included in the pricing structure must be considered. This margin reflects the value added by Hilma Biocare through innovation, quality assurance, and brand reputation.
What Factors Influence Pricing for Hilma Biocare Products?
Several factors can influence the pricing of Hilma Biocare’s offerings:
-
Volume and Minimum Order Quantity (MOQ): Larger orders often lead to reduced unit costs. Buyers should negotiate MOQs that align with their needs to maximize cost-effectiveness.
-
Specifications and Customization: Custom formulations or specific packaging requests can lead to higher prices. It is essential for buyers to clearly communicate their requirements to avoid unexpected costs.
-
Quality Certifications: Products that comply with international quality standards may carry a premium. Buyers should evaluate the certifications relevant to their markets to ensure compliance and quality assurance.
-
Supplier Factors: The reputation and reliability of Hilma Biocare as a supplier can influence pricing. A well-regarded supplier may command higher prices due to perceived quality and reliability.
-
Incoterms: Understanding the International Commercial Terms (Incoterms) is crucial for international buyers. These terms dictate who bears the costs and risks associated with shipping, which can significantly impact the total cost of procurement.
How Can Buyers Optimize Costs When Sourcing from Hilma Biocare?
For international B2B buyers, particularly from regions such as Africa, South America, the Middle East, and Europe, optimizing costs involves several strategies:
-
Effective Negotiation: Buyers should leverage their purchasing power when negotiating prices, especially for bulk orders. Building a strong relationship with Hilma Biocare can lead to better pricing and terms.
-
Total Cost of Ownership (TCO): Consider the TCO rather than just the purchase price. This includes shipping, storage, and potential costs related to product returns or regulatory compliance.
-
Pricing Nuances: Be aware of pricing fluctuations due to market conditions, exchange rates, and regulatory changes. Staying informed can help buyers time their orders more effectively.
-
Exploring Local Partnerships: Establishing relationships with local distributors familiar with Hilma Biocare’s products can streamline logistics and potentially reduce costs associated with import duties and tariffs.
Disclaimer on Indicative Prices
While pricing examples from Hilma Biocare indicate a range of costs for various products, these are subject to change based on market conditions, order volume, and negotiation outcomes. Buyers should consult directly with Hilma Biocare for the most accurate and up-to-date pricing information tailored to their specific needs.
Alternatives Analysis: Comparing hilma biocare With Other Solutions
When evaluating solutions for pharmaceutical products, it’s essential to consider various alternatives that meet the specific needs of your business. In this section, we will compare Hilma Biocare with two viable alternatives—Apex Biologics and Genix Pharma. Each option presents unique advantages and challenges, allowing B2B buyers to make informed decisions based on their operational requirements.
| Comparison Aspect | Hilma Biocare | Apex Biologics | Genix Pharma |
|---|---|---|---|
| Performance | High-quality generics, GMP certified | Focus on biopharmaceuticals, innovative products | Wide range of generic medications |
| Cost | Reasonably priced for high-quality products | Moderate pricing, premium for innovation | Competitive pricing with bulk options |
| Ease of Implementation | User-friendly ordering process, good customer support | Some complexity in product integration | Straightforward ordering, less customer support |
| Maintenance | Minimal, with a focus on quality control | Higher due to ongoing innovation | Low, standard operational procedures |
| Best Use Case | General pharmaceuticals and steroids | Advanced biological products for niche markets | Broad-spectrum generics for cost-sensitive markets |
What are the Advantages and Disadvantages of Apex Biologics?
Apex Biologics specializes in biopharmaceuticals and innovative drug development, making it an excellent choice for companies looking for cutting-edge solutions. The performance of their products is generally high, which can be appealing for firms focused on research and development. However, the cost can be on the higher side, especially for those seeking innovative products. Additionally, integrating Apex’s offerings may involve complexities due to their advanced nature, making it less suitable for businesses looking for straightforward solutions.
What are the Pros and Cons of Genix Pharma?
Genix Pharma offers a broad range of generic medications, which can be a cost-effective solution for businesses that prioritize affordability. Their competitive pricing and extensive product range cater to various market needs, making them an attractive option for companies in regions with budget constraints. However, the trade-off may come in the form of reduced customer support and a less innovative product lineup compared to Hilma Biocare and Apex Biologics. This could be a drawback for businesses that require more personalized service or cutting-edge products.
How Can B2B Buyers Choose the Right Solution?
Choosing the right pharmaceutical solution involves assessing your specific needs against the strengths and weaknesses of each option. Hilma Biocare stands out for its quality generics and user-friendly processes, making it ideal for businesses that prioritize both quality and ease of access. Apex Biologics is suitable for those in search of innovative products and are willing to invest more, while Genix Pharma appeals to budget-conscious buyers looking for a wide range of options. Ultimately, the decision should align with your operational goals, budget constraints, and product requirements to ensure the best fit for your organization.
Essential Technical Properties and Trade Terminology for hilma biocare
What Are the Key Technical Properties of Hilma Biocare Products?
When evaluating Hilma Biocare products, several critical technical properties stand out, particularly for B2B buyers in the pharmaceutical sector. Understanding these properties can enhance decision-making and ensure compliance with quality standards.
-
Active Pharmaceutical Ingredient (API) Concentration
The concentration of the API in a product directly affects its efficacy and safety. For instance, Hilma Biocare’s injectable products, such as testosterone enanthate (250 mg/ml), must adhere to strict dosage specifications. This concentration is crucial for healthcare providers and distributors to ensure that patients receive the intended therapeutic effect without exceeding safe limits. -
Formulation Type
Hilma Biocare offers various formulation types, including injectables, oral tablets, and peptides. Each formulation has unique characteristics that influence absorption rates and bioavailability. For example, injectable products may provide faster onset of action compared to oral tablets. B2B buyers must understand these differences to match product offerings with patient needs and regulatory requirements. -
Packaging Integrity
The integrity of packaging is vital for maintaining the product’s stability and efficacy. Hilma Biocare has recently transitioned to dark glass vials to protect light-sensitive products. Effective packaging prevents contamination and degradation, which is essential for ensuring product quality throughout the supply chain. Buyers should prioritize suppliers that adhere to high packaging standards to minimize risk. -
GMP Compliance
Good Manufacturing Practice (GMP) compliance is a non-negotiable standard in the pharmaceutical industry. Hilma Biocare operates under stringent GMP regulations, ensuring that products are manufactured consistently and controlled to quality standards. This compliance reassures B2B buyers about the reliability and safety of the products, critical for maintaining their own business reputations. -
Stability and Shelf Life
Understanding the stability and shelf life of pharmaceutical products is essential for inventory management and regulatory compliance. Hilma Biocare ensures that all products undergo rigorous testing to determine their shelf life under various conditions. This information helps B2B buyers plan for procurement and manage stock levels effectively, minimizing waste and maximizing profitability.
What Common Trade Terms Should B2B Buyers Know in the Pharmaceutical Industry?
Navigating the pharmaceutical supply chain involves familiarizing oneself with industry-specific jargon. Here are key terms that are particularly relevant for buyers considering Hilma Biocare products.
-
Minimum Order Quantity (MOQ)
MOQ refers to the smallest quantity of a product that a supplier is willing to sell. Understanding MOQ is essential for B2B buyers as it can impact inventory costs and cash flow management. Hilma Biocare may set MOQs based on production capabilities, which can influence purchasing decisions. -
Request for Quotation (RFQ)
An RFQ is a document issued when a buyer wants to receive pricing information from suppliers. It typically includes details about the required products, quantities, and delivery timelines. Utilizing RFQs can lead to competitive pricing and better supplier relationships, allowing buyers to secure favorable terms with Hilma Biocare. -
Original Equipment Manufacturer (OEM)
An OEM refers to a company that produces parts or equipment that may be marketed by another manufacturer. For B2B buyers, understanding OEM relationships is crucial when considering branded versus generic products. Hilma Biocare primarily focuses on generic pharmaceuticals, which can provide cost-effective alternatives. -
Incoterms
International Commercial Terms (Incoterms) are a set of rules that define the responsibilities of buyers and sellers in global trade. They clarify aspects like shipping costs, insurance, and delivery points. Familiarity with Incoterms is critical for B2B buyers in ensuring that contracts with Hilma Biocare are clear and enforceable, minimizing potential disputes. -
Pharmaceutical Grade
This term indicates that the product meets specific purity and quality standards suitable for pharmaceutical use. Buyers should verify that Hilma Biocare’s products are pharmaceutical grade to ensure compliance with regulatory standards and patient safety.
By understanding these properties and terms, B2B buyers can make informed decisions that align with their business objectives and regulatory requirements, ensuring successful collaborations within the pharmaceutical landscape.
Navigating Market Dynamics and Sourcing Trends in the hilma biocare Sector
What Are the Current Market Dynamics and Key Trends Affecting the Hilma Biocare Sector?
The global pharmaceutical landscape is rapidly evolving, driven by a myriad of factors including technological advancements, regulatory changes, and shifting consumer demands. For international B2B buyers, particularly those in regions such as Africa, South America, the Middle East, and Europe, understanding these dynamics is crucial for informed sourcing decisions.
One of the most significant trends is the rise of digital transformation within the pharmaceutical supply chain. Technologies such as blockchain are enhancing transparency and traceability, which are essential for compliance with stringent regulations. Furthermore, the adoption of e-commerce platforms is streamlining procurement processes, allowing buyers from different regions to access Hilma Biocare products more conveniently.
In addition, there is a growing demand for generic pharmaceuticals, which are more affordable and accessible to a broader population. Hilma Biocare’s focus on high-quality generics positions it favorably in markets where cost-effectiveness is paramount. Another emerging trend is the increasing importance of personalized medicine, where products are tailored to meet specific health needs, further driving innovation within the Hilma Biocare portfolio.
How Is Sustainability and Ethical Sourcing Impacting B2B Buyers in the Hilma Biocare Sector?
As sustainability becomes a pivotal concern for global industries, the pharmaceutical sector is no exception. B2B buyers are increasingly prioritizing suppliers that demonstrate a commitment to ethical sourcing and environmental stewardship. For Hilma Biocare, this means adopting sustainable practices throughout the production process, from raw material sourcing to packaging.
The environmental impact of pharmaceuticals is significant, with waste management and carbon emissions being critical issues. Buyers are now looking for partners who can provide eco-friendly certifications and materials. For instance, Hilma Biocare’s transition to dark glass vials not only enhances product safety but also reflects a commitment to sustainability by reducing plastic use.
Moreover, ethical supply chains that ensure fair labor practices and responsible sourcing are becoming non-negotiable for many buyers. This shift toward sustainability not only fulfills corporate social responsibility goals but also resonates with consumers who are increasingly environmentally conscious. Therefore, B2B buyers in the Hilma Biocare sector must evaluate potential partners based on their sustainability practices to align with evolving market expectations.
What Is the Brief Evolution and History of Hilma Biocare Relevant to B2B Context?
Hilma Biocare has established itself as a prominent player in the pharmaceutical industry over the past eight years, focusing on the development and manufacture of generics aimed at improving quality of life. With operations in 47 countries and a diverse product portfolio that includes injectables, oral tablets, and peptides, the company has built a reputation for quality and affordability.
The commitment to rigorous clinical testing and adherence to Good Manufacturing Practices (GMP) underscores Hilma Biocare’s dedication to producing high-quality medicaments. This evolution reflects a broader trend in the pharmaceutical industry where quality assurance and regulatory compliance are paramount, making Hilma Biocare an attractive partner for B2B buyers seeking reliable suppliers in the competitive global marketplace.
By navigating these market dynamics and trends, international B2B buyers can make strategic sourcing decisions that not only enhance their product offerings but also align with their business values and customer expectations.
Frequently Asked Questions (FAQs) for B2B Buyers of hilma biocare
-
How do I ensure the quality of Hilma Biocare products before purchasing?
To ensure the quality of Hilma Biocare products, request documentation that confirms adherence to Good Manufacturing Practices (GMP) and any relevant certifications. Additionally, consider conducting your own quality assurance checks by ordering samples before committing to larger orders. Engaging in direct communication with Hilma Biocare’s representatives can also provide insights into their manufacturing processes and quality control measures, ensuring that the products meet your standards. -
What are the minimum order quantities (MOQs) for Hilma Biocare products?
The minimum order quantities (MOQs) for Hilma Biocare products can vary based on the specific product and your location. It is advisable to reach out directly to their sales team for detailed information on MOQs applicable to your order. Understanding the MOQ will help you plan your inventory and budget effectively, ensuring that you can meet market demand without excess stock. -
What payment terms does Hilma Biocare offer for international buyers?
Hilma Biocare typically offers flexible payment terms tailored to the needs of international buyers. Common options include bank transfers, credit card payments, and potentially PayPal for smaller transactions. It’s essential to discuss payment terms directly with their sales team to understand any upfront deposits, payment schedules, and available credit options, ensuring a smooth transaction process. -
How can I customize my order of Hilma Biocare products?
Customization options for Hilma Biocare products may be available depending on your specific needs and order size. To explore customization, contact their customer service or sales team to discuss your requirements. They may offer options such as private labeling, packaging variations, or specific formulations tailored to your market. Early communication can help align your needs with their production capabilities. -
What logistics and shipping options are available for international orders?
Hilma Biocare provides various logistics and shipping options for international orders, which may include express and standard shipping methods. The choice of shipping will depend on your urgency and budget. It’s crucial to confirm shipping costs, delivery timelines, and customs regulations with their shipping department to ensure a seamless delivery process to your region. -
How does Hilma Biocare handle product returns and exchanges?
Hilma Biocare has a return policy that typically allows for product returns or exchanges under specific conditions, such as product defects or shipping errors. To initiate a return, you should contact their customer service within a designated timeframe after receiving your order. Ensure you have your order details ready, as this will expedite the process and help you understand any applicable terms or conditions. -
What certifications should I look for when sourcing Hilma Biocare products?
When sourcing products from Hilma Biocare, look for certifications such as GMP, ISO, or other relevant pharmaceutical quality standards. These certifications indicate adherence to high-quality manufacturing processes and regulatory compliance. Requesting these documents during your supplier vetting process can help ensure you are partnering with a reputable manufacturer committed to product safety and efficacy. -
How can I evaluate the reliability of Hilma Biocare as a supplier?
To evaluate the reliability of Hilma Biocare as a supplier, consider researching their market presence, customer reviews, and industry reputation. Engaging in direct communication with their sales representatives can also provide insights into their responsiveness and customer service. Additionally, seeking testimonials from other B2B clients can help gauge their reliability and performance, ensuring you make an informed decision.
Important Disclaimer & Terms of Use
⚠️ Important Disclaimer
The information provided in this guide, including content regarding manufacturers, technical specifications, and market analysis, is for informational and educational purposes only. It does not constitute professional procurement advice, financial advice, or legal advice.
While we have made every effort to ensure the accuracy and timeliness of the information, we are not responsible for any errors, omissions, or outdated information. Market conditions, company details, and technical standards are subject to change.
B2B buyers must conduct their own independent and thorough due diligence before making any purchasing decisions. This includes contacting suppliers directly, verifying certifications, requesting samples, and seeking professional consultation. The risk of relying on any information in this guide is borne solely by the reader.
Top 7 Hilma Biocare Manufacturers & Suppliers List
1. Hilma Biocare – Pharmaceutical Solutions
Domain: hilmabiocare.com
Registered: 2014 (11 years)
Introduction: Hilma Biocare offers a range of pharmaceutical products including: 1. Injectable products 2. Oral tablets 3. Erectile dysfunction treatments 4. Post Cycle Therapy (PCT) products 5. Peptides 6. HGH (Human Growth Hormone) products 7. Semaglutide (newly introduced product) The company emphasizes the quality of its products, which are manufactured under GMP standards, ensuring they are produced in ste…
2. Hilma Biocare – Semaglutide (Ozempic)
Domain: hilmabiocare.shop
Introduction: {“products”:[{“name”:”Hilma Biocare – Semaglutide (Ozempic)”,”dosage”:”2 mg/vial”,”original_price”:”€64.99″,”current_price”:”€44.99″},{“name”:”HGH Somatropin Liquid”,”dosage”:”5 x KITS (500IU)”,”original_price”:”€1,000.00″,”current_price”:”€699.99″},{“name”:”Hilma Biocare – Nandrolone Decanoate (Deca Durabolin)”,”dosage”:”250 mg/ml”,”price”:”€39.99″},{“name”:”Hilma Biocare – Oxandrolone (Anavar)”,…
3. Hilma Biocare – Key Product
Domain: woodbridgebrewingco.com
Registered: 2022 (3 years)
Introduction: Hilma Biocare offers a diverse range of supplements targeting various health needs, including immune support, digestive health, stress management, and cognitive enhancement. The company prioritizes high-quality, scientifically-backed ingredients, ensuring effective and safe formulations. Key product features include innovative packaging for nutrient preservation, advanced manufacturing techniques …
4. Hilma Biocare – Testosterone Enanthate
Domain: hilmabiocareshop.com
Registered: 2023 (2 years)
Introduction: {“products”:[{“name”:”Hilma Biocare Testosterone Enanthate”,”delivery”:”Delivery from 6 to 12 days”,”price”:”€ 37.00″,”status”:”Sold out”},{“name”:”Hilma Biocare Nandrolone Decanoate”,”delivery”:”Delivery from 6 to 12 days”,”price”:”€ 40.00″,”status”:”Available”},{“name”:”Hilma Biocare Sustanone”,”delivery”:”Delivery from 6 to 12 days”,”price”:”€ 33.00″,”status”:”Available”},{“name”:”Hilma Biocare…
5. Hilmabiocare – Testosterone Enanthate
Domain: hilmabiocare.eu
Introduction: [{‘name’: ‘TESTOSTERONE ENANTHATE’, ‘form’: ‘Injectable’, ‘dosage’: ‘250 MG/ML – 10 ML’, ‘price’: ‘28,99 €’}, {‘name’: ‘OXANDROLONE (ANAVAR)’, ‘form’: ‘Oral’, ‘dosage’: ’10 MG/TAB. – 100 TABS’, ‘price’: ‘38,99 €’}, {‘name’: ‘METHENOLONE ENANTHATE (PRIMOBOLAN)’, ‘form’: ‘Injectable’, ‘dosage’: ‘100 MG/ML – 10 ML’, ‘price’: ‘64,99 €’}, {‘name’: ‘TESTOSTERONE CYPIONATE’, ‘form’: ‘Injectable’, ‘dosage…
6. Hilma Biocare – Growth Hormone Injection
Domain: indiamart.com
Registered: 1996 (29 years)
Introduction: {“Product Name”:”Hilma Biocare 3.33 mg Growth Hormone Injection”,”Price”:”₹ 600/Vial”,”Seller”:”Shree DKR”,”Location”:”Nagpur, Maharashtra”,”Brand”:”Hilma Biocare”,”Packaging Type”:”Box”,”Usage/Application”:”Clinical”,”Composition”:”Human Growth Hormone Injection”,”Packaging Size”:”Box”,”Prescription/Non-Prescription”:”Non-Prescription”,”Availability”:”In Stock”,”Shipping Information”:”Ships from …
7. Hilma Biocare – Muscle Fusion X
Domain: de.pinterest.com
Registered: 2009 (16 years)
Introduction: Hilma Biocare specializes in providing the best sports pharmacy products on the market, including Muscle Fusion X.
Strategic Sourcing Conclusion and Outlook for hilma biocare
Hilma Biocare represents a compelling opportunity for international B2B buyers seeking reliable, high-quality pharmaceutical products. With a strong emphasis on generic medicines, this company has established a reputation for producing effective treatments that meet rigorous GMP standards. The strategic sourcing of Hilma Biocare products not only ensures access to quality medications but also offers competitive pricing, which is crucial for markets in Africa, South America, the Middle East, and Europe.
For buyers in these regions, engaging with Hilma Biocare means tapping into a network that prioritizes both product efficacy and affordability. The introduction of innovative formats, such as the HGH Somatropin Liquid PEN, exemplifies the company’s commitment to enhancing user experience while maintaining high standards of health and safety.
As we look to the future, the landscape of pharmaceutical sourcing is poised for growth, driven by increased demand for quality healthcare solutions. Now is the time for international buyers to explore partnership opportunities with Hilma Biocare, leveraging their extensive product portfolio to meet market needs effectively. Reach out today to discover how Hilma Biocare can enhance your supply chain and elevate the quality of healthcare in your region.